Iron Iii Chloride Reacts With Sodium Carbonate . the reactions of the aqueous ions iron (ii) and iron (iii) with ammonia, sodium hydroxide and sodium carbonate are described and explained. enter an equation of an ionic chemical equation and press the balance button. this video is the practical demonstration of the reaction of iron (iii). The balanced equation will be calculated along. the hexaaquairon (iii) ion is sufficiently acidic to react with the weakly basic carbonate ion. If you add sodium carbonate solution to a solution of. iron (iii) chloride reacts with sodium carbonate in a double displacement reaction. Write the balanced molecular equation,. Aqueous iron (iii) chloride ( f e c l 3) and sodium carbonate ( n a 2 c o 3) solution yields aqueous sodium. recall that in the mole ratio, 2 moles of iron (iii) chloride will react for every 3 moles of sodium carbonate.
from www.numerade.com
the reactions of the aqueous ions iron (ii) and iron (iii) with ammonia, sodium hydroxide and sodium carbonate are described and explained. Aqueous iron (iii) chloride ( f e c l 3) and sodium carbonate ( n a 2 c o 3) solution yields aqueous sodium. the hexaaquairon (iii) ion is sufficiently acidic to react with the weakly basic carbonate ion. If you add sodium carbonate solution to a solution of. Write the balanced molecular equation,. The balanced equation will be calculated along. recall that in the mole ratio, 2 moles of iron (iii) chloride will react for every 3 moles of sodium carbonate. iron (iii) chloride reacts with sodium carbonate in a double displacement reaction. enter an equation of an ionic chemical equation and press the balance button. this video is the practical demonstration of the reaction of iron (iii).
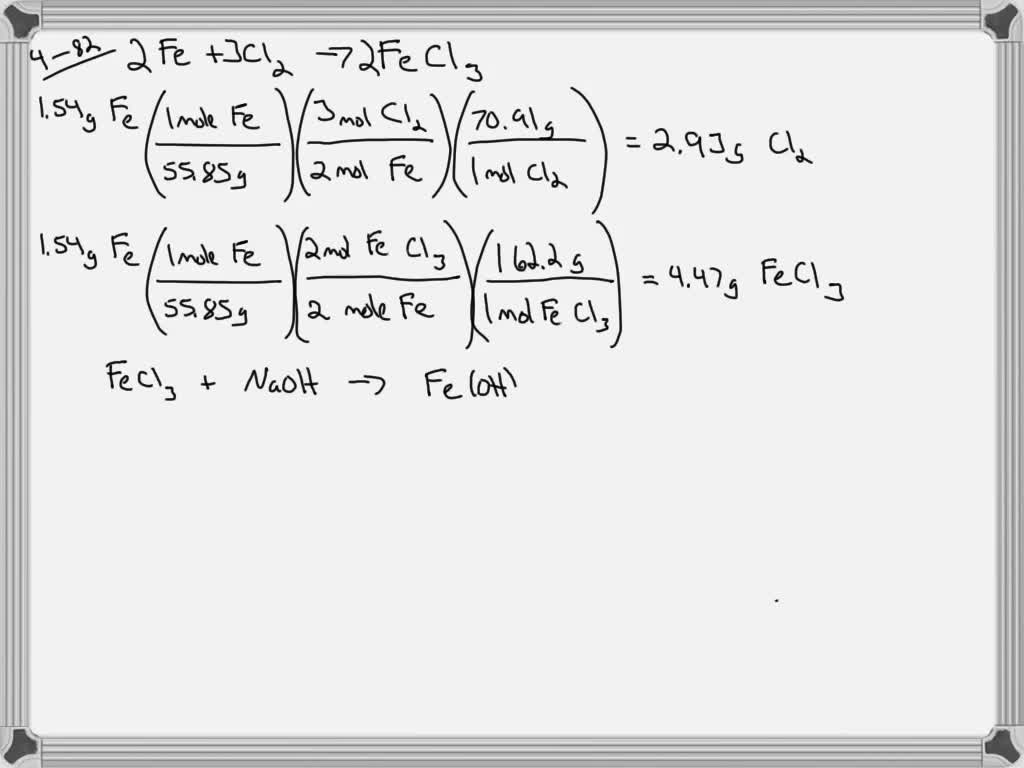
SOLVEDIron(III) chloride is produced by the reaction of iron and
Iron Iii Chloride Reacts With Sodium Carbonate iron (iii) chloride reacts with sodium carbonate in a double displacement reaction. Write the balanced molecular equation,. If you add sodium carbonate solution to a solution of. Aqueous iron (iii) chloride ( f e c l 3) and sodium carbonate ( n a 2 c o 3) solution yields aqueous sodium. the reactions of the aqueous ions iron (ii) and iron (iii) with ammonia, sodium hydroxide and sodium carbonate are described and explained. this video is the practical demonstration of the reaction of iron (iii). iron (iii) chloride reacts with sodium carbonate in a double displacement reaction. recall that in the mole ratio, 2 moles of iron (iii) chloride will react for every 3 moles of sodium carbonate. enter an equation of an ionic chemical equation and press the balance button. the hexaaquairon (iii) ion is sufficiently acidic to react with the weakly basic carbonate ion. The balanced equation will be calculated along.
From stock.adobe.com
Chemical reactionPipette 0.25 M solution of iron(III) chloride (FeCl3 Iron Iii Chloride Reacts With Sodium Carbonate the hexaaquairon (iii) ion is sufficiently acidic to react with the weakly basic carbonate ion. recall that in the mole ratio, 2 moles of iron (iii) chloride will react for every 3 moles of sodium carbonate. the reactions of the aqueous ions iron (ii) and iron (iii) with ammonia, sodium hydroxide and sodium carbonate are described and. Iron Iii Chloride Reacts With Sodium Carbonate.
From www.numerade.com
SOLVED You react 2.33 g of iron (III) chloride with 50.0 mL phosphate Iron Iii Chloride Reacts With Sodium Carbonate Aqueous iron (iii) chloride ( f e c l 3) and sodium carbonate ( n a 2 c o 3) solution yields aqueous sodium. enter an equation of an ionic chemical equation and press the balance button. iron (iii) chloride reacts with sodium carbonate in a double displacement reaction. Write the balanced molecular equation,. recall that in. Iron Iii Chloride Reacts With Sodium Carbonate.
From www.numerade.com
SOLVED How much excess reactant is left over when 50 mL of 0.250 M Iron Iii Chloride Reacts With Sodium Carbonate enter an equation of an ionic chemical equation and press the balance button. Aqueous iron (iii) chloride ( f e c l 3) and sodium carbonate ( n a 2 c o 3) solution yields aqueous sodium. this video is the practical demonstration of the reaction of iron (iii). Write the balanced molecular equation,. the reactions of. Iron Iii Chloride Reacts With Sodium Carbonate.
From slideplayer.com
Chapter 4 Chemical Reactions ppt download Iron Iii Chloride Reacts With Sodium Carbonate enter an equation of an ionic chemical equation and press the balance button. Aqueous iron (iii) chloride ( f e c l 3) and sodium carbonate ( n a 2 c o 3) solution yields aqueous sodium. iron (iii) chloride reacts with sodium carbonate in a double displacement reaction. the reactions of the aqueous ions iron (ii). Iron Iii Chloride Reacts With Sodium Carbonate.
From www.bartleby.com
Answered The Iron metal reacts with chlorine gas… bartleby Iron Iii Chloride Reacts With Sodium Carbonate iron (iii) chloride reacts with sodium carbonate in a double displacement reaction. the hexaaquairon (iii) ion is sufficiently acidic to react with the weakly basic carbonate ion. Aqueous iron (iii) chloride ( f e c l 3) and sodium carbonate ( n a 2 c o 3) solution yields aqueous sodium. this video is the practical demonstration. Iron Iii Chloride Reacts With Sodium Carbonate.
From slideplayer.com
Chemical Bonding III. Ionic Compounds. ppt download Iron Iii Chloride Reacts With Sodium Carbonate If you add sodium carbonate solution to a solution of. the reactions of the aqueous ions iron (ii) and iron (iii) with ammonia, sodium hydroxide and sodium carbonate are described and explained. recall that in the mole ratio, 2 moles of iron (iii) chloride will react for every 3 moles of sodium carbonate. enter an equation of. Iron Iii Chloride Reacts With Sodium Carbonate.
From www.youtube.com
Iron III Chloride Reaction With Potassium Thiocyanate (FeCl3 + KSCN Iron Iii Chloride Reacts With Sodium Carbonate Write the balanced molecular equation,. the reactions of the aqueous ions iron (ii) and iron (iii) with ammonia, sodium hydroxide and sodium carbonate are described and explained. iron (iii) chloride reacts with sodium carbonate in a double displacement reaction. The balanced equation will be calculated along. this video is the practical demonstration of the reaction of iron. Iron Iii Chloride Reacts With Sodium Carbonate.
From www.researchgate.net
What is the chemical difference between these iron III chloride Iron Iii Chloride Reacts With Sodium Carbonate iron (iii) chloride reacts with sodium carbonate in a double displacement reaction. the reactions of the aqueous ions iron (ii) and iron (iii) with ammonia, sodium hydroxide and sodium carbonate are described and explained. recall that in the mole ratio, 2 moles of iron (iii) chloride will react for every 3 moles of sodium carbonate. The balanced. Iron Iii Chloride Reacts With Sodium Carbonate.
From stock.adobe.com
Chemical reactionRusty red iron(III) hydroxide precipitate (Fe(OH)3 Iron Iii Chloride Reacts With Sodium Carbonate If you add sodium carbonate solution to a solution of. the hexaaquairon (iii) ion is sufficiently acidic to react with the weakly basic carbonate ion. recall that in the mole ratio, 2 moles of iron (iii) chloride will react for every 3 moles of sodium carbonate. The balanced equation will be calculated along. the reactions of the. Iron Iii Chloride Reacts With Sodium Carbonate.
From www.bartleby.com
The reaction of iron metal and chlorine gas to give iron(III) chloride Iron Iii Chloride Reacts With Sodium Carbonate this video is the practical demonstration of the reaction of iron (iii). enter an equation of an ionic chemical equation and press the balance button. iron (iii) chloride reacts with sodium carbonate in a double displacement reaction. Write the balanced molecular equation,. The balanced equation will be calculated along. Aqueous iron (iii) chloride ( f e c. Iron Iii Chloride Reacts With Sodium Carbonate.
From www.numerade.com
SOLVED In the reaction to form iron(III) chloride, 10.0 grams of iron Iron Iii Chloride Reacts With Sodium Carbonate Aqueous iron (iii) chloride ( f e c l 3) and sodium carbonate ( n a 2 c o 3) solution yields aqueous sodium. the hexaaquairon (iii) ion is sufficiently acidic to react with the weakly basic carbonate ion. iron (iii) chloride reacts with sodium carbonate in a double displacement reaction. recall that in the mole ratio,. Iron Iii Chloride Reacts With Sodium Carbonate.
From www.alamy.com
Iron (III) hydroxide precipitation. Test tube containing sodium Iron Iii Chloride Reacts With Sodium Carbonate If you add sodium carbonate solution to a solution of. iron (iii) chloride reacts with sodium carbonate in a double displacement reaction. enter an equation of an ionic chemical equation and press the balance button. the reactions of the aqueous ions iron (ii) and iron (iii) with ammonia, sodium hydroxide and sodium carbonate are described and explained.. Iron Iii Chloride Reacts With Sodium Carbonate.
From www.numerade.com
SOLVEDThe reaction of iron metal and chlorine gas to give iron(III Iron Iii Chloride Reacts With Sodium Carbonate recall that in the mole ratio, 2 moles of iron (iii) chloride will react for every 3 moles of sodium carbonate. this video is the practical demonstration of the reaction of iron (iii). the hexaaquairon (iii) ion is sufficiently acidic to react with the weakly basic carbonate ion. Write the balanced molecular equation,. iron (iii) chloride. Iron Iii Chloride Reacts With Sodium Carbonate.
From www.coursehero.com
[Solved] When aqueous an solutions of iron(III) sulfate and sodium Iron Iii Chloride Reacts With Sodium Carbonate If you add sodium carbonate solution to a solution of. iron (iii) chloride reacts with sodium carbonate in a double displacement reaction. the reactions of the aqueous ions iron (ii) and iron (iii) with ammonia, sodium hydroxide and sodium carbonate are described and explained. this video is the practical demonstration of the reaction of iron (iii). . Iron Iii Chloride Reacts With Sodium Carbonate.
From www.slideserve.com
PPT Oral assessment PowerPoint Presentation, free download ID3444733 Iron Iii Chloride Reacts With Sodium Carbonate If you add sodium carbonate solution to a solution of. the hexaaquairon (iii) ion is sufficiently acidic to react with the weakly basic carbonate ion. the reactions of the aqueous ions iron (ii) and iron (iii) with ammonia, sodium hydroxide and sodium carbonate are described and explained. this video is the practical demonstration of the reaction of. Iron Iii Chloride Reacts With Sodium Carbonate.
From brainly.in
aqueous iron chloride and sodium carbonate solution yields aqueous Iron Iii Chloride Reacts With Sodium Carbonate the hexaaquairon (iii) ion is sufficiently acidic to react with the weakly basic carbonate ion. iron (iii) chloride reacts with sodium carbonate in a double displacement reaction. this video is the practical demonstration of the reaction of iron (iii). If you add sodium carbonate solution to a solution of. The balanced equation will be calculated along. . Iron Iii Chloride Reacts With Sodium Carbonate.
From www.numerade.com
SOLVED In water, iron(III) chloride reacts with sodium hydroxide Iron Iii Chloride Reacts With Sodium Carbonate iron (iii) chloride reacts with sodium carbonate in a double displacement reaction. recall that in the mole ratio, 2 moles of iron (iii) chloride will react for every 3 moles of sodium carbonate. Write the balanced molecular equation,. Aqueous iron (iii) chloride ( f e c l 3) and sodium carbonate ( n a 2 c o 3). Iron Iii Chloride Reacts With Sodium Carbonate.
From www.scientificlabs.ie
Iron(III) chloride, , sublimed 7011225G SIGMA ALDRICH SLS Ireland Iron Iii Chloride Reacts With Sodium Carbonate recall that in the mole ratio, 2 moles of iron (iii) chloride will react for every 3 moles of sodium carbonate. Aqueous iron (iii) chloride ( f e c l 3) and sodium carbonate ( n a 2 c o 3) solution yields aqueous sodium. this video is the practical demonstration of the reaction of iron (iii). . Iron Iii Chloride Reacts With Sodium Carbonate.